3 brands on FSA’s list of valid novel food applications (so far)
Just three CBD manufacturers on the FSA’s list of valid applications awaiting authorisation.
The Food Standards Agency (FSA) has revealed the three CBD brands which have had success so far under the novel foods regulation. The publication of the list comes almost three weeks after the deadline for submission.
Chanelle McCoy Health, Cannabis Pharma and Health Innovations Ltd. are the three manufacturers and suppliers which now sit at the top of the FSA’s list of valid applications.
Each of the three manufacturers are in the oils and capsules product categories. However, while validated, each manufacturer must still await final authorisation which is not guaranteed.
The agency, which has overseen the implementation of CBD as a novel food since it was first announced in January 2018, says the products listed on these applications ‘should be allowed to stay on the market until a decision on their authorisation has been made.’
Health Innovations is a UK-registered company which produces a range of CBD products under the name 4MP Technologies.
Cannabis Pharma is located in Czech Republic, while Chanelle McCoy Health is based in Ireland.
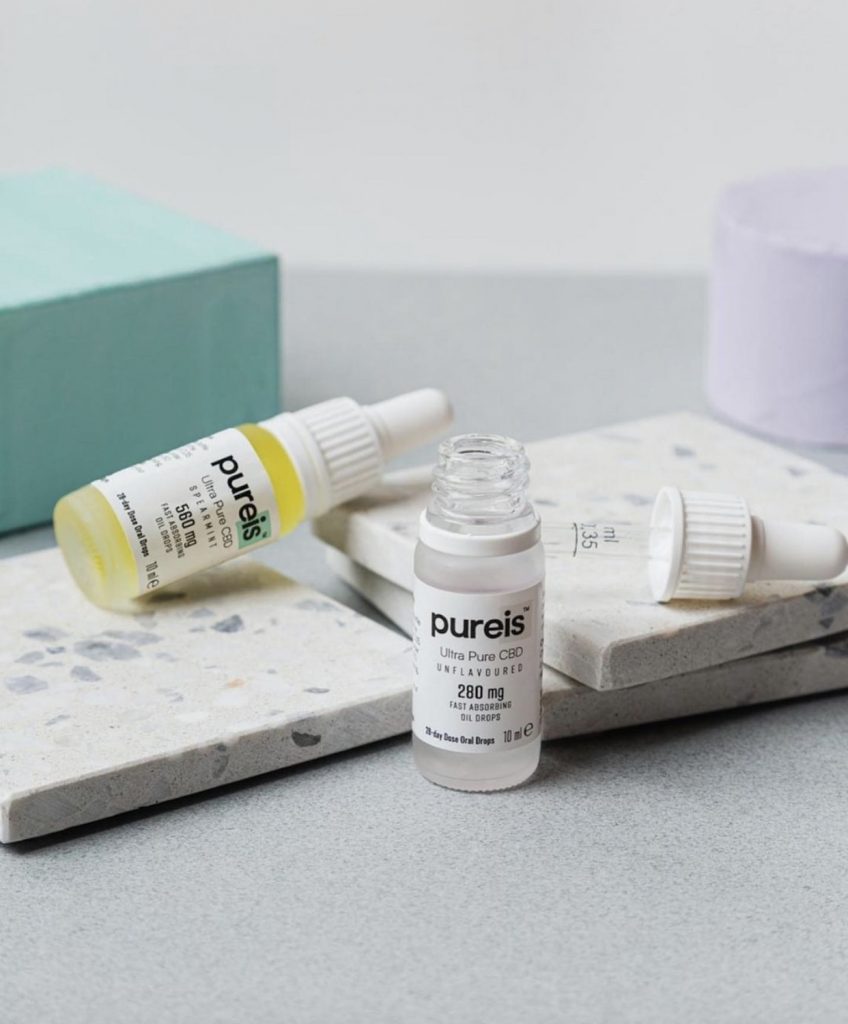
The latter produces the Pureis Ultra Pure CBD range, which is believed to be the first brand with dual validation under the novel foods regulation at an EU and UK level. The range is now primed to be the first legal CBD food supplement on sale backed by clinical trial data.
Founder and entrepreneur Chanelle McCoy is a judge on the Irish television adaptation of popular shark tank series, Dragon’s Den and the wife of jockey, Sir AP McCoy.
The novel foods regulation has been imposed at a UK and EU level on all edible CBD products, as experts warn there is insufficient history of CBD extracts being present in the food chain prior to May 1997.
In England and Wales, the Food Standards Agency imposes the application process while it is up to each local authority to enforce the rules. Scotland and Northern Ireland implement their own protocols under the guidelines.
The deadline for applications to be filed to the FSA was March 31, 2021, with many brands, manufacturers and suppliers now waiting to hear where they stand.
Once an application has been made, it is considered for validation, prior to being authorised. Only once a product has been authorised can it be deemed fully compliant, and therefore legal, under the novel foods regulation.
The FSA says among the steps CBD companies must complete is a risk assessment.
“Validation requires checking that an application contains all information required by law to allow it to proceed to the authorisation process. This information includes evidence of a risk assessment. If any of this information is missing, the application cannot be legally validated.
“It is important to note that validation is not the same as authorisation. There is no guarantee that a validated application will be authorised.”
Updates on the process for CBD brands on sale in the UK can be found on the official agency website.










